 |
Normal-pulse polarography (NPP) Differential-pulse polarography (DPP) Cyclic voltammetry (CV) Anodic-stripping voltammetry Chronoamperometry (CA) Rotating Disc Electrode |
Voltammetry encompasses a collection of techniques that induces an electrochemical reaction by applying a potential, and measures the resulting current. In order to analyze both the voltage and the resulting current, three electrodes are employed in an electrochemical cell. The three electrodes include the working, auxillary, and reference electrodes which, when connected through a voltmeter, can yield results about ionic concentrations, conductivity, and diffusion. In the most common voltammetry method, polarography, the current induced by the applied voltage is described by the Ilkovic equation:
where n is the number of electrons per molecule in the redox reaction, C is the concentration of electroactive species (mmol/L), D is the diffusion coefficient of electroactive species (m^2/s), m is the flow rate of Hg (mg/s), and t is the drop interval (s).
To learn more about polarography and other voltammetric methods, click on the following links:
 |
Normal-pulse polarography (NPP) Differential-pulse polarography (DPP) Cyclic voltammetry (CV) Anodic-stripping voltammetry Chronoamperometry (CA) Rotating Disc Electrode |
The results of voltammetry experiments are plotted as applied voltage versus the resultant current. Click on this voltammoggram below to go to run a computer program that models cyclic voltammetry experiments at a large Interface between Two Immiscible Electrolyte Solutions (ITIES) for various reversible ion transfer reactions.
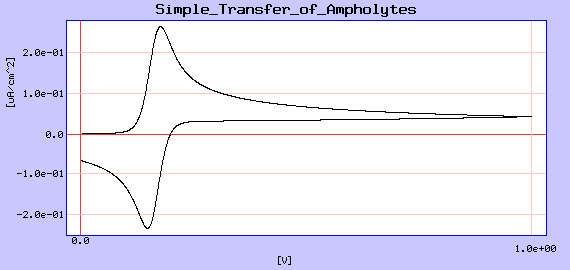
Voltammetric experiments can be done on the undergraduate level in the bioanalytical chemistry laboratory. One voltammetry experiment uses Hg drop electrodes to analyze the concentration of certain metal ions in water. Click here for more experiments that can be done in the bioanalytical chemistry laboratory.
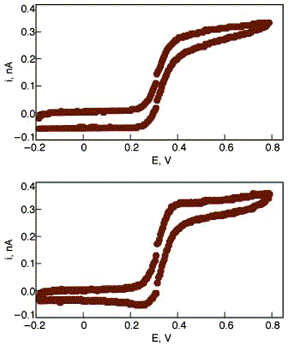
Voltammetry can be used to determine the concentration of analyte in a solution, or to determine the diffusion coefficient of various analytes. On a nanostructure level, voltammetry has recently been used to analyze microvials. These voltammoggrams come from a review article analyzing the use of cyclic voltammetry for nanoscale experiments. Click on the graphs to read more about this technique.
More reference articles on the bioanalytical applications of voltammetry
Return to Bioanalytical Homepage