|
|
|
 |
|
|
|
 |
We will study two main sources of air pollution in this lesson:
pollution from coal-fired and natural gas electrical power plants
and pollution from automobiles. The main pollutants from all of
these sources include NOx, CO, hydrocarbons and particulate matter.
Coal-fired plants have additional pollutants that include SO2,
radioactive compounds, heavy metals and solids like fly ash. We
will briefly review the pollutants particular to coal fired power
plants and then move on to study the chemistry of NOx, CO and
hydrocarbons in more detail.
Electricity can be produced in a variety of ways. The key point is that other forms of energy must be converted into electricity. In the United States, most electricity comes from burning fossil fuels. That conversion process is never completely clean; to the contrary, it is typically a major source of environmental problems.
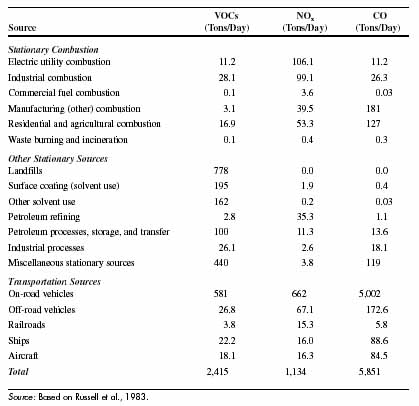
In general, the environmental impacts of electric power depend on the technology and primary energy source used to produce electricity. The most common energy source for power generation in the United States is coal, which provided 52 percent of all electricity in 1997 (USDOE, 1998a). The next most important energy source is uranium used by nuclear power plants, providing 18 percent of U.S. electricity. The remaining 30 percent is shared by natural gas (14 percent), hydroelectric power (10 percent), oil (3 percent), and miscellaneous renewable sources (3 percent) consisting of geothermal, biomass, municipal solid waste (including landfill gas), wind, and solar energy. The figure below shows the historical trend in the U.S. energy mix for electric power in the U.S.
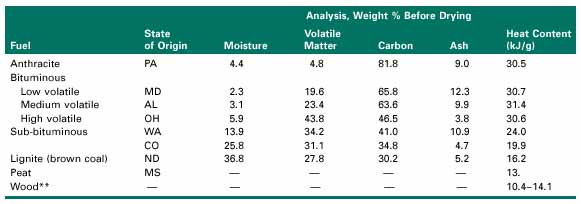
The environmental concerns about fossil fuel power plants today center primarily around the atmospheric emissions of carbon dioxide (CO2), sulfur dioxide (SO2), nitrogen oxides (NOx), and particulate matter (PM). The table below shows the quantities of these emissions released per kilowatt-hour of electricity generated in the United States by different types of power plants. Note that these emission rates reflect the current differences in power generation efficiency using different types of fuels and technologies.
Because of the potential impacts of these pollutants on human health and the environment, federal and state regulations limit the amounts that can be released from any particular facility. In general, federal emission limits apply to newly constructed plants, whereas state and local regulations cover existing power plants. An exception is the set of federal rules for acid rain control, which applies to all facilities. Examples of federal emission limits are given in the table below.
Natural gas releases 42 percent less CO2 than coal while supplying the same amount of energy. Different fuel compositions will give slightly different results, but in all cases natural gas emits substantially less CO2 than coal per unit of energy supplied, provided that the conversion efficiencies are the same. This environmental benefit is one of the reasons that natural gas has become increasingly attractive as a fuel for electric power generation.
One of the most important impurities in fossil fuels is sulfur. Sulfur occurs in the mineral form as pyrite (a compound of iron and sulfur, also known as "fool's gold" because of its bright yellow color) and also in the organic form within the molecular structure of coal. Oil and natural gas also contain sulfur compounds when extracted from the ground. However, most of these impurities are removed in oil refineries and gas treatment plants, which clean and refine the raw fuel before it is distributed for use. Coal, on the other hand, undergoes little or no processing for sulfur removal before it is burned. Thus the highest levels of sulfur impurities usually are found in coal. When coal is burned, a small amount of the sulfur, typically 2 to 5 percent of the total (depending on coal type), is retained in the solid ash particles. Most of the sulfur (95 percent or more) is oxidized to gaseous sulfur dioxide SO2:
This SO2 is a major contributor to acid rain and a source of adverse
health effects. A very small amount (less than 1 percent) of the
SO2 formed during combustion may be further oxidized to SO3, which
reacts with water vapor in the combustion gas to form gaseous
sulfuric acid (H2SO4). When released to the atmosphere, this gas
condenses into fine liquid droplets that remain suspended in the
air. This acid aerosol is another source of the environmental
impacts associated with sulfur impurities in fossil fuels.
Another major impurity in fossil fuels is mineral matter, commonly called ash. This solid incombustible material includes compounds of iron, silicon, and other common elements of the earth's crust. Indeed, most natural elements-including toxic heavy metals-may be found in the ash of fossil fuels, especially coal. Because oil and natural gas are refined before use, they contain little or no ash when combusted. Coal, on the other hand, is relatively high in ash, including rock and mineral matter torn from the ground in the mining process. Some of this material is separated from coal prior to combustion. Still, roughly 10 percent of the coal burned in power plants is incombustible material (akin to the gray ash residue left in your charcoal barbecue). Unless preventive measures are taken, coal combustion releases into the atmosphere large amounts of this ash (also known as flyash because it "flies" out of the chimney). All modern power plants, however, have environmental control systems that remove nearly all the ash particles entrained in the combustion gas stream. Solids that escape collection may pose an environmental concern. Such particulate matter emissions are among the criteria air pollutants.
Another major class of air pollutants from fossil fuel combustion is oxides of nitrogen (NOx). NOx consists mainly of nitric oxide (NO) and nitrogen dioxide (NO2) formed from high-temperature reactions between oxygen and nitrogen. NOx can be formed solely from the molecular nitrogen and oxygen in air, as well as from the atomic nitrogen and oxygen impurities in fuel. Because its formation during the combustion process is very complex, the amount of NOx usually must be determined experimentally. For oil and coal, NOx emissions per unit of fuel energy are usually higher than for natural gas combustion. The higher NOx levels are due mainly to the presence of fuel-bound nitrogen, which is readily converted to NO when the fuel is combusted. The amount of "fuel-bound NOx" is typically two to three times greater than the "thermal NOx" produced only from the reactions between N2 and O2 in air. Though NOx constitutes only a small fraction of the total combustion products, its environmental impacts are nonetheless significant.
Three basic approaches are available to reduce the environmental impacts of power plants. The first involves technological measures to control or remove a pollutant before it is released to the environment.
The second approach is the application of "green design"
principles to increase the efficiency of electric power generation.
Higher efficiency means less primary energy is needed to generate
a desired amount of electricity; environmental impacts are reduced
proportionally. This approach is most fruitful when designing
new facilities, although modest efficiency gains often can be
found at many existing plants. Green design also includes pollution
prevention approaches such as innovations that produce useful
by-products rather than solid wastes.
The third approach to pollution abatement involves selecting and
utilizing cleaner energy sources and alternative technologies
with lower environmental impacts. Similar in concept to green
design, this option applies to situations where more than one
technology can do a given job-in this case, producing a given
amount of electricity.
Combustion of gasoline, like any chemical reaction, gives off
products. Complete combustion of a hydrocarbon releases only water
and carbon dioxide. Water is pretty safe stuff, of course, and
carbon dioxide, while contributing to greenhouse warming and global
climate change, is at least non-toxic to breathe. But due to incomplete
combustion, side reactions with atmospheric components other than
oxygen, and reactions of impurities in the gasoline, your car's
tailpipe also releases some other substances that are not so benign.
In this lesson you will learn what those substances are, what
part of the combustion process generates them, and how they are
regulated. You will learn about the health effects of these pollutants
and start to consider the potential role of oxygenate additives
in altering the pollution from automobiles.
Sources of Air Pollutants
As you know, the two major products from combustion of automobile
fuel are CO2 and H2O. Life as we know it on this planet would
be impossible if we did not have enough of these two molecules.
However, too much of a good thing may not always be beneficial,
since both are greenhouse gases. In this module you will focus
on compounds that are emitted in much lower quantity, but have
known human health effects. These compounds include nitrogen
oxides, hydrocarbons, and carbon monoxide. These
compounds have health effects of their own and can also combine
in the atmosphere to produce other hazardous gases, such as ozone
(O3), a component of the unpleasant pollution mixture known as
smog. Once you understand how combusting fuels in automobiles
produces these compounds and the tradeoffs involved in reducing
the emissions of each, you will assess whether oxygenated fuels
should be used to improve air quality.
While this lesson focuses on the pollutants added to the atmosphere
by automobiles, the major sources of each of these air pollutants
are in fact natural. Hydrocarbons are naturally produced by many
sources. For example, methane is generated by anaerobic decay
of organic material in areas such as wetlands and rice paddies,
and terpenes are released by trees and crops. Carbon monoxide
is mainly generated by the natural atmospheric oxidation of methane.
Nitrogen oxides form by the bacterial action of soil microbes
and subsequent atmospheric reactions of these compounds. Figures
1A-C represent the worldwide average of the amounts of each pollutant
emitted from all sources.
Figure 1A: Sources of global emissions of hydrocarbons
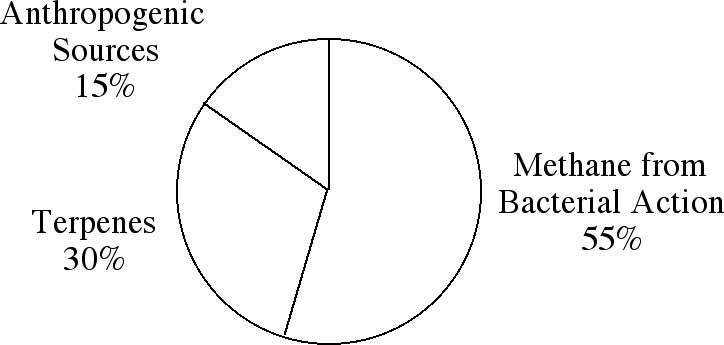
Figure 1B: Sources of global emissions of carbon monoxide.
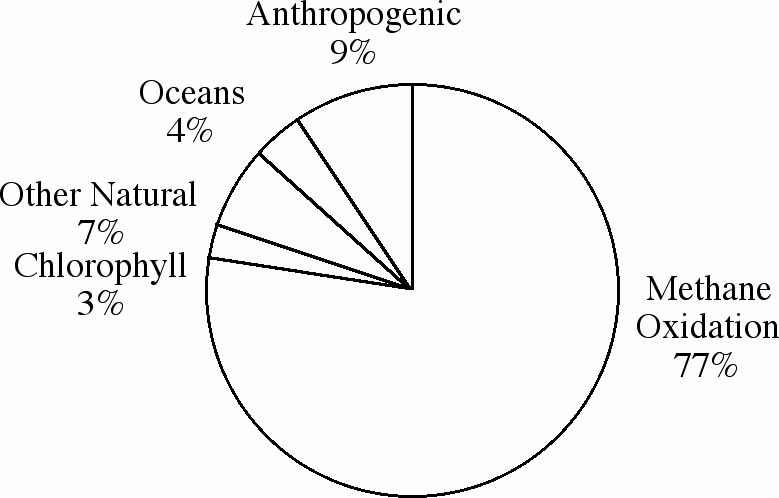
Figure 1C: Sources of global emissions of nitrogen oxides.
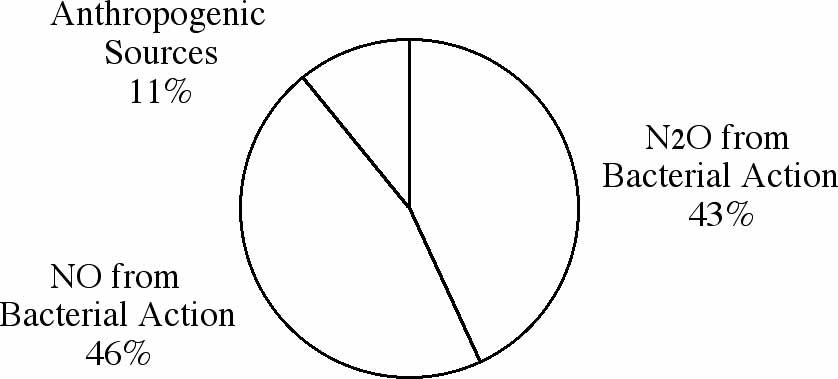
Former President Ronald Reagan once remarked that most air pollution
is caused by trees. Though he was not quite correct, as you can
see from the figures, remarks like his have made many people wonder
why we should worry about anthropogenic, or human-generated,
sources. Although the anthropogenic pollution sources are smaller
in amount, they are of great concern due to their localized concentrations.
Natural pollutants are often generated over wider areas, and tend
to become distributed relatively uniformly by atmospheric circulation
and mixing. Anthropogenic pollutants, however, tend to be emitted
in a concentrated space and time, and thus may be emitted at much
higher net rates than natural pollutants in a particular place.
Pollutants emitted by automobiles are concentrated in areas of
high population and traffic density. There is not enough time
for the pollutants to disperse before more pollutants are added
daily, keeping the average level in the local atmosphere high.
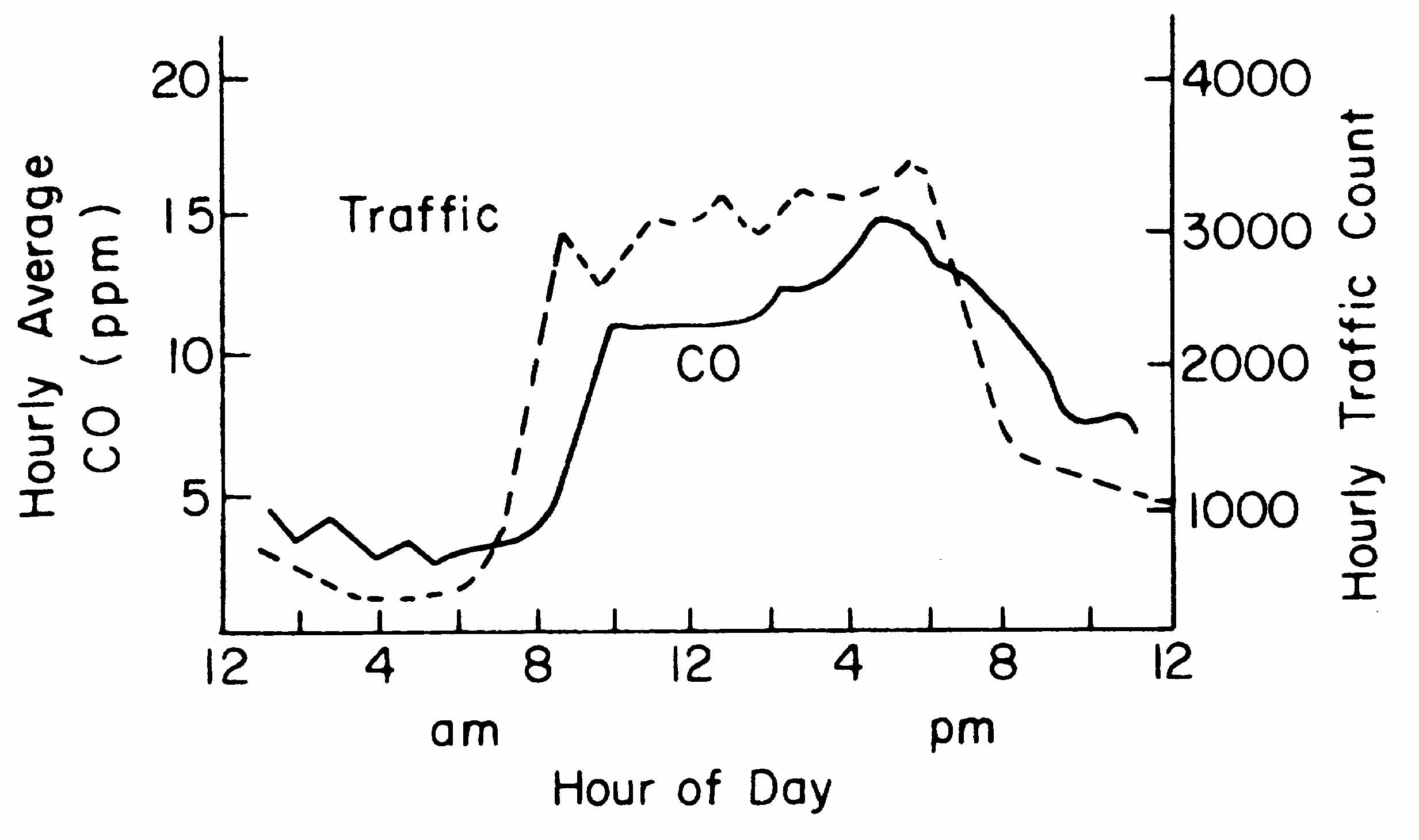
For example, more than 95% of the carbon monoxide in the atmosphere
of metropolitan areas results from human activities. Local concentrations
can be fifty to one hundred times greater than global average
concentrations. This can overwhelm the capacity of natural mechanisms
to cleanse the air. Figure 1D illustrates this effect by comparing
the hourly average CO concentrations with traffic patterns in
downtown New York City. These concentrations of CO represent the
levels above the background of natural emissions (Figure 1D).
Production, health effects and control of carbon monoxide
Carbon monoxide (CO) is produced in vehicle exhaust when there
is either insufficient time for reaction or insufficient oxygen
for complete combustion of gasoline in the engine. You will investigate
this situation in detail later in your studies. At high concentrations,
those above 100 ppm (parts per million), carbon monoxide is lethal.
At lower concentrations, adverse human health effects are less
well documented but still serious. Carbon monoxide binds to the
hemoglobin in blood very efficiently, thus inhibiting binding
of oxygen. The reduced blood capacity for oxygen leads to acute
effects of CO exposure such as headache, fatigue and dizziness.1
Nonetheless, a large class of citizens voluntarily inhale large
concentrations of CO multiple times each day. Can you guess who
they might be?
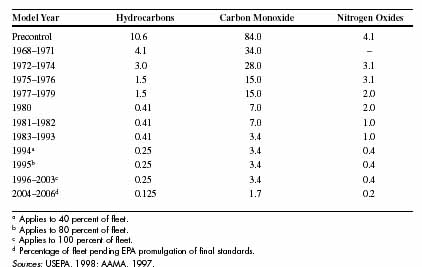
The Clean Air Act of 1970 required automobile manufacturers to
reduce pollutants resulting from fuel combustion. Later amendments
to the Act have further reduced allowable concentrations (see
Table 1B-1). One very effective response to these requirements
has been the introduction of catalytic converters. The
primary function of automobile catalytic converters is to convert
CO to CO2 in the presence of air and a metal catalyst. Since catalytic
converters were first installed in U.S. automobiles in 1975, the
number of cars in the U.S. has doubled, but atmospheric CO concentrations
have decreased by 40%2-a remarkable achievement of chemistry and
automotive technology.
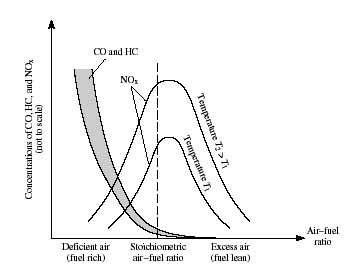
Production, health effects and control of nitrogen oxides
Nitrogen oxides are produced in engines as a side effect of
fuel combustion, the reaction at high temperature of the nitrogen
and oxygen both naturally present in the air needed to combust
the fuel. Because air is needed to react the fuel itself, there
is no way to avoid this additional reaction, an issue you will
explore in detail later in this module. Transportation accounts
for about half of total anthropogenic NOx emissions, with power
plants and other stationary combustion sources the next major
source.
For a chemist, the nitrogen oxides include NO, NO2, and N2O. Atmospheric
scientists, however, are most concerned by the toxic and highly
reactive compounds NO and NO2, and refer to these collectively
as "NOx", meaning x can be equal to 1 or 2. These two
compounds can be grouped because they rapidly interconvert in
the atmosphere and thus have a joint role in producing smog. Nitric
oxide (NO) is the major form produced directly by combustion,
but NO2 is more prevalent in the atmosphere. Nitrous oxide (N2O,
laughing gas) is classified separately because it is much less
chemically reactive and does not participate in smog-forming chemistry.
Nitrogen oxides affect air quality in a number of ways. Atmospheric
oxidation of NO leads to higher oxides of nitrogen and eventually
nitric acid, a significant source of acid rain, second only to
the sulfur oxides. The sunlight-catalyzed reaction in the atmosphere
between unburned hydrocarbon molecules and NOx leads to photochemical
smog, the brown haze often seen above large cities. One product
of these reactions is ozone, a pollutant in the lower atmosphere
that is a respiratory irritant. It is ironic that anthropogenic
emissions are responsible for both an excess of ozone in the troposphere
(lower atmosphere) and ozone depletion in the stratosphere
(middle atmosphere).
Compared to the significant progress made in reducing CO, attempts
to reduce NOx levels have been less successful. New catalytic
converters do include a catalyst for reducing NO back to N2 and
O2, but this reducing chemistry is challenging in the presence
of the oxidation catalyst needed to remove CO. However, even though
the number of automobiles has doubled since 1975, NOx levels have
remained essentially constant in the last 15 years. Contributing
factors include improved catalytic converters, better engine design,
and improved gasoline formulations. Despite these gains, reducing
NOx emissions and smog is still a formidable challenge for auto
makers and pollution control agencies.
Production, health effects and control of hydrocarbons
Hydrocarbons are a large class of compounds formed from carbon
and hydrogen, and thus are sometimes collectively designated "CHx".
In the context of air pollution, this category includes unburnt
and partially burnt components of gasoline, whether released at
the tailpipe by incomplete combustion or evaporated from fuel
tanks, filling stations, and refineries. Transportation accounts
for about half the total hydrocarbon emissions. The term "volatile
organic compounds" or VOCs is often used interchangeably.
This term is broader and recognizes the fact that partially burnt
hydrocarbons are not, strictly speaking, hydrocarbons, because
they incorporate some oxygen in their structures. Examples of
this group include formaldehyde (HCHO) and acetaldehyde (CH3CHO).
Atmospheric hydrocarbons at current ambient levels (i.e.
the amounts present in the air we breathe) are a health concern
less because of their own hazards than because of their role in
forming other, more hazardous compounds. Research indicates that
even at much higher concentrations, aliphatic, or linear-chain,
hydrocarbons will not exhibit adverse health affects. However,
aromatic, or cyclic, hydrocarbons pose a greater threat.
The simplest aromatic hydrocarbon, benzene, is found in gasoline,
and is a noted carcinogen. Aromatic vapors are also irritants
when inhaled. Moreover, many compounds formed by atmospheric oxidation
of hydrocarbons, including the aldehydes and irritating smog components
such as peroxyacetylnitrate (PAN) and ozone, exhibit their own
adverse health effects.
Production of ozone in the troposphere requires sunlight, NOx
and VOCs. Thus both NOx and VOCs are controlled by environmental
agencies. Newer catalytic converters include a catalyst to oxidize
partially burnt hydrocarbons completely to CO2, along with the
CO. Snug-fitting fuel caps, vapor recovery devices on fuel pumps,
and inspection and maintenance of fuel lines and tanks are measures
that can help reduce evaporative emission of hydrocarbons. The
Clean Air Act will mandate further reductions if a high percentage
of seriously polluted cities remain smoggy.
A final hazard of VOCs, particularly the more polar compounds
such as the aldehydes, is their tendency to stick together in
the atmosphere to form tiny liquid particles called aerosols.
The aerosols will also incorporate acidic material from the NOx.
These particles, as well as soot and other particles directly
emitted by vehicles, are responsible for the reduced visibility
or "haze" that is one of smog's signature effects. They
are a respiratory hazard, particularly the smaller particles less
than 10 microns, which are less likely to be trapped by nose hairs
and the mucous membranes before entering the lungs.
Particulate emissions are receiving increasing attention from
atmospheric scientists, toxicologists, and pollution regulators.
Because the complex chemistry and physics of their formation are
not yet well understood, you will not study particulates in detail
in this module. However, you should keep this additional pollutant
in mind as you consider the broader issues of air pollution from
automobiles.
Table 1E: Vehicle exhaust emissions standards in grams per mile for U.S. passenger cars (excluding California, which has its own standards) (3)
Oxygenated fuel additives are combustible compounds
that contain oxygen in their molecular structure. Examples of
the most common oxygenated fuel additives are given in Table 1E.
There is ongoing debate about whether such additives help to reduce
air pollution, a debate you will learn more about as you work
through this lesson.
Most oxygenated fuel additives are also octane enhancers, providing
higher octane ratings when mixed with gasoline. Oxygenated were
first introduced to the US as octane enhancers and as domestic
substitutes for oil during the Arab oil embargo of the early 1970s.
The octane numbers of gasoline mixtures used in the United States
typically range from 85 to 94. The range of octane levels available
depends on geographic region and manufacturer. You might assume
that the higher prices charged for higher octane fuel correlate
with either greater fuel efficiency or greater fuel performance.
However, this isn't always true.
The EPA mandates that oxygenated gasolines include at least 2.7% oxygen by mass in fuels sold in carbon monoxide non-attainment areas. A non-attainment area is one that is not currently meeting EPA standards for the particular pollutant in question. The requirement for oxygenated fuels in CO non-attainment areas is known as the Wintertime Oxy-Fuel program, because carbon monoxide tends to accumulate when the air is not well-mixed. This occurs more often in the winter, when temperature inversions can occur, trapping a stagnant layer of air near the surface. These weather conditions are also more common in western cities, particularly those bordered by mountains, which tend to help trap the inversions. In ozone non-attainment areas, the EPA requires at least 2.0% oxygen by mass. This program is known as the Summer Reformulated Gasoline (RFG) program. Based on what you know already about smog formation, why do you think this program is required in the summer?
Table 1E: Oxygenated Fuel Additives and Amounts Needed to Meet EPA Standard of 2.7% O by Mass for CO Non-Attainment Areas
|
|
|
(g/mL) |
|
|
| ethanol | CH3CH2OH |
|
||
| methanol | CH3OH |
|
|
|
| MTBE | (CH3)3COCH3 |
|
||
| ETBE | (CH3)3COCH2CH3 |
|
||
| TAME | (CH3)3CCH2OCH3 |
|
||
| TBA | (CH3)3COH |
|
Ozone (O3) is a reactive gas composed of three oxygen atoms, in contrast to normal molecular oxygen (O2). Most of the earth's atmospheric ozone (8590 percent) is found in the stratosphere-the portion of the atmosphere between about 10 and 45 kilometers (km) altitude. In the stratosphere, ozone plays a crucial role in absorbing ultraviolet radiation emitted by the sun. The remaining 1015 percent of atmospheric ozone can be found in the troposphere-the lower part of the atmosphere extending from the earth's surface to an altitude of approximately 10 km.
Ozone is a trace atmospheric gas with natural concentrations in
the clean troposphere of approximately 3040 parts per billion
(ppb). If the entire atmospheric ozone volume were collapsed to
a pressure of one atmosphere, it would form a layer only 3 mm
thick. Because ozone exists in such small concentrations in the
atmosphere, human activities are more apt to cause changes in
its concentration.
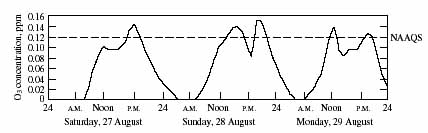
High ozone concentrations exceeding roughly 100 ppb have been shown to be associated with adverse effects on the human respiratory system. The effects are more significant for people who exercise. Acute effects, such as changes in lung capacity and function, have been observed in some people after exposures of less than an hour. In some cases the effects persist for many hours or days. High ozone concentrations also can injure trees and agricultural crops as well as damage certain materials.
Expressing the carbon content of a fuel in relation to the fuel energy content gives a direct link between energy consumption and carbon emissions. The ratio of a fuel 's carbon content to its energy content defines the carbon intensity :
|
|
kJ/L |
L/L-gasoline |
|
|
|
|
|
|
|
|
|
|
|
|
|
(Liquefied petroleum gas, primarily propane) |
|
|
|
|
|
|
|
|
|
|
|
(Compressed natural gas, primarily methane, at 200 atm) |
|
|
|
(Liquefied natural gas, primarily methane) |
|
|
Once the fuel heating value is determined (based either on
HHV or LHV), the corresponding carbon intensity is calculated
as:
The fraction of carbon in fuel may be specified on either a mass
basis or a molar basis, consistent with the units used for heating
value.
|
|
(Weight % C) |
(kJ/g) |
(gC/MJ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1) Excerpted from Rubin, Engineering & the Environment, Chapter 5.
2) Excerpted from Drossman, Laursen and Tikkanen, Air Pollution Module, ChemLinks
3) Excerpted from Rubin, Engineering & the Environment, Chapter 3.
4) Diagrams from Rubin, Engineering & the Environment, Chapters 3 & 5.
|
|
|
|
|
|