The Importance of pH
by Ben O'Donnell
Activity =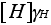
where 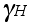 is the activity coefficient
of Hydrogen (H).
is the activity coefficient
of Hydrogen (H).
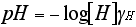
A pH meter measures the negative
logarithm of the hydrogen ion activity, not just its concentration.
Ions in solution do not act according to their concentrations,
which would be ideal, the activity coefficient is the deviation
of this behavior from the ideal.(Harris 1999, 178).
Buffers are solutions that resist change in pH even when acids
or bases are added or when they are diluted. They are very important
to many bioanalytical procedures, from things like solution stability
to analyte-ion binding due to pH as in capillary electrophoresis(Harris
1999, 178).
For some information on the biological
significance of pH, go to the link listed below.
How a pH meter works(another
description, from the web):
A pH meter consists of a reference electrode
coupled to a working electrode. The reference electrode contains
a saturated solution of a solid and a salt-for instance AgCl-which
maintains a constant potential. The two electrodes measure the
electric potential across a glass membrane. The glass membrane
is located at the bottom of the pH electrode, with the reference
electrode and working electrode contained within one tube. The
working electrode is in contact with the glass electrode via a
saturated solution of HCl and AgCl. The reference electrode is
in contact with the working electrode through a salt bridge like
solution of KCl and AgCl. This allows the two electrodes to interact
electrically , which is how the electric potential of the glass
membrane is measured. The glass membrane itself consists of a
hydrated gel layer on the inner and outer surfaces, separated
by a dry glass layer. H+ from the solution can diffuse into the
gel membrane where it reacts with metal cations, which is known
as ion-exchange equilibrium. The H+ then is the only ion that
can be measured by this type of electrode, because it is the only
ion that can bind significantly to the glass membrane(Harris 1999,
178). This is a picture of one kind of glass electrode, it has
all of the basic parts described above, although it is hard to
see.

The potential difference off the inner and
outer surface of the glass membrane is measured in relation to
the hydrogen ion and chloride ion concentrations on the inside
of the glass membrane, since the concentrations of these ions
are constant on the inside of the electrode. The only variable
then is the concentration of hydrogen on the outside of the glass
membrane. The equation for this potential is as follows:
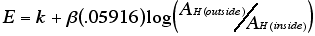
The value of ß (electromotive efficiency) is accepted as
1 generally. The pH meter is then calibrated by using solutions
with known values of pH(Harris 1999, 178).
Some Review articles
from ChemAbstracts:
Studies of vanadate-organic ligand systems
using potentiometry and NMR spectroscopy
Author: Pettersson, Lage, and others Source: ACS Symp. Ser. American
Chemical Society 711, no. Vanadium Compounds (1998): 30-50
The next two articles are older, but the field of pH and pH meters
is not exactly cutting edge chemistry.
Behavior of the glass electrode and other
pH-responsive electrodes in biological media Author: Bates, Roger G., and others Source: Ann. N.
Y. Acad. Sci. 148, no. 1 (1968): 67-80
Sensitive measurements of changes of hydrogen ion concentration
Author: Chance, Britton, and others Source: Methods Enzymol. 10
(1967): 641-50
Review articles and
procedures from JChemEd online:
K. L. Cheng: A New Concept for pH-Potential Calculations
This paper discusses the concept of pH-potential calculations
and indicates that the pH electrode is a capacitor, not a half-cell
as currently believed. Citation: Cheng, K. L. A New Concept for
pH-Potential Calculations J. Chem. Educ. 1999 76 1029.
Mary M. Walczak, Deborah A. Dryer, Dana D. Jacobson, Michele G.
Foss, and Nolan T. Flynn: pH Dependent Redox Couple: An Illustration
of the Nernst Equation
Electrochemical reactions depending on pH are ideal for illustrating
the Nernst equation for undergraduate laboratories. An experiment
utilizing the hydroquinone/quinone redox was developed which follows
the potentials of the anodic and cathodic cyclic voltammetric
waves as a function of solution pH. The 1mM hydroquinone solutions
are prepared in phosphate/acetate mixed buffers with pH between
1-6. Cyclic voltammograms were obtained for each solution. As
solution pH increases, the anodic and cathodic potentials shift
cathodically as predicted by the Nernst equation. Plots of peak
potential vs. pH were linear and showed that the hydroquinone/quinone
redox reaction involves two electrons. J. Chem. Educ. 1997 74
1195.
Lai, S. T. F.; Burkhart, R. D. Evaluation of acid-base indicator
constants using a limited pH range. J. Chem. Educ. 1976 53
500.
Finlayson, A. C. The pH range of the Mohr titration for chloride
ion can be usefully extended to 4-10.5 (TF). J. Chem. Educ.
1992 69 559.
Dole, Malcolm. The early history of the development of the
glass electrode for pH measurements (SBS). J. Chem. Educ.
1980 57 134.
Brower, Harold E. A glass electrode pH meter using a vacuum
tube voltmeter. J. Chem. Educ. 1963 40 85.
Jackman, Donald C. A recipe for the preparation of a pH 7.00
calibration buffer. J. Chem. Educ. 1993 70 853.
Vitz, Ed; Betts, Thomas A. LIMSport (V): pH Data Acquisition:
An Inexpensive Probe Calibration Software (CS). J. Chem. Educ.
1994 71 412.
Other Web sites dealing
with pH and electrochemistry:
UBC
WWW Site: A good site but pretty basic, no pun intended-it
deals with the biological importance of pH, which was mentioned
very briefly above.
Innovative
Sensors: An excellent site that deals with a wide range of
pH questions
A site demonstrating the process of calibrating
a pH electrode.
Tulane
University: A fun site where you are playing in the pH pool,
need I say more?
pH
Site at ?: This is a good site as well, with good presentation.
Back to Bioanalytical Home
Page
