- What is the Difference Between a Covalent and Ionic Bond?
- Why does table salt (NaCl) in solution allow
the light bulb to glow while the sugar solution does not? Make sure to
include the types of bonds in your answers. What happens when you subject
table salt and sugar to heat? What experiment is a better indication of
bond energy?
- What is a Chemical Reaction?
- What evidence can you compile for a chemical reaction vs. a physical
change?
- What is the Structure of Water and Why Does it Assume That Shape?
- Assemble a molecular model of water using the
model kits provided. What is the shape of the water molecule? Why? Now
draw the structure on paper, including all electrons. Is water a molecule?
Is NaCl a molecule?
What Are Some Implications of the Shape of Water?
- Hydrogen bonding
- Using your models, demonstrate the nature of
hydrogen bonding by combining your models with three other people. Do this
for the liquid phase and the gas phase. Now, use the structure of water
that you drew to explain what is happening on paper.
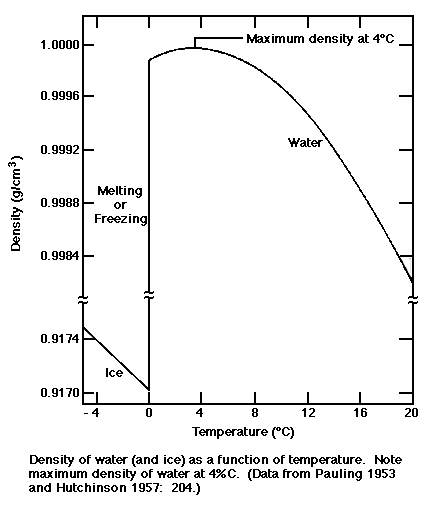
- Density of water
- What is the meaning of density? How would you
measure the density of water in the laboratory? The density of water as
a function of temperature is given in the following figure (Ref 1). using ideas
about the molecular structure of water and hydrogen bonding, provide an
explanation of the shape of the curve. Does ice float or sink in water?
Is this explained by the diagram. Can you use the diagram to predict how
much of the ice is submerged in water?
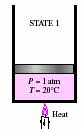
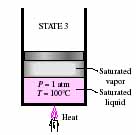
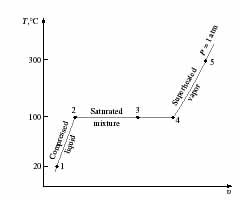
- Specific heat, Heat capacity and Latent heat of water and ice
- In the following P-V diagram and figures below
it, why the line is sloped from 1-2? What is the meaning of slope with
relation to specific heat and heat capacity? What is the difference between
specific heat and heat capacity? Why is the line flat from 2-4? Why is
it sloped again from 4-5? If the diagram had a solid phase, how would you
draw such a phase? What is the difference between sensible heat and latent
heat?
|
|
|
|
|
A summary of some physcal properties of water are provided below (Reference 2).
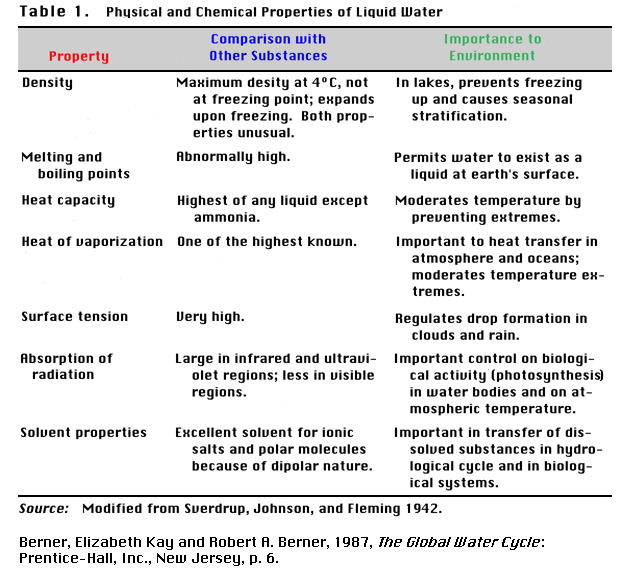
Figure references:
1. http://hercules.gcsu.edu/~sdatta/home/teaching/hydro/slides/density_t.gif
2. http://hercules.gcsu.edu/~sdatta/home/teaching/hydro/slides/prop_h2o.gif