|
Aqueous concentration units
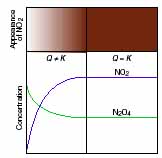
Review of chemical equilibriumEquilibrium applies to the extent of a reaction, the concentration of product that has appeared given unlimited time, or when no further change is observed. Countless experiments with chemical systems have shown that, in a state of equilibrium, the concentrations of reactants and products no longer change with time. This apparent cessation of chemical activity occurs because all reactions are reversible. Let's examine a reacting chemical system at the macroscopic and molecular levels to see how reversibility gives rise to the equilibrium state. The system consists of two gases, colorless dinitrogen tetraoxide and brown nitrogen dioxide: If we introduce a small amount of N2O4(l) into a sealed flask kept at 100°C, the liquid immediately vaporizes (bp = 21°C) and the gas begins to turn pale brown. The color slowly darkens, but after a few moments, no further color change can be seen. As we close in on the molecular level, a much more active scene unfolds. The N2O4 molecules fly wildly throughout the flask, some splitting into two NO2 molecules. As time passes, more N2O4 molecules decompose and the concentration of NO2 rises. As observers in the macroscopic world, we see the flask contents darken because NO2 is reddish-brown. As the number of N2O4 molecules decreases, N2O4 decomposition slows. At the same time, increasing numbers of NO2 molecules collide and combine, so N2O4 re-formation speeds up. Eventually, N2O4 molecules decompose as fast as NO2 molecules combine. The system has reached equilibrium: reactant and product concentrations stop changing because the forward and reverse rates have become equal, Thus, a system at equilibrium continues to be dynamic at the molecular level, but we see no further net change because changes in one direction are balanced by changes in the other. At a particular temperature, the product and reactant concentrations do not change when the system reaches equilibrium. Therefore, the ratio of their concentrations must be a constant. We'll use the rate laws for the N2O4-NO2 system to derive this constant. At equilibrium, the rate of the forward reaction equals the rate of the reverse reaction Since, in this case, both forward and reverse reactions are elementary steps, we can use the balanced equation to write rate laws for them: where kfwd and krev are the forward and reverse rate constants, respectively. By rearranging, we set the ratio of the rate constants equal to the ratio of the concentration terms:
The ratio of constants gives rise to a new overall constant called the equilibrium constant (K): The equilibrium constant K is a number whose value is equal to the ratio of rate constants. In addition, and most importantly, K is equal to a particular ratio of equilibrium product and reactant concentration terms at a particular temperature. We examine this idea closely in the next section and show that it holds as well for overall reactions made up of several elementary steps. Although the concentrations of reactants and products are constant in a system at equilibrium, this does not mean that the system contains 50% products and 50% reactants. It is the opposing rates that are equal at equilibrium, not necessarily the concentrations. Indeed, different reactions, even at the same temperature, have a wide range of concentrations at equilibrium-from almost all reactant to almost all product-and therefore, they have a wide range of equilibrium constants. Here are three specific examples: 1. Small K. If a reaction goes very little toward product before reaching equilibrium, K will be small. For example, the oxidation of nitrogen barely proceeds at 1000 K: 2. Large K. Conversely, if the reaction reaches equilibrium with virtually no reactant remaining, we say it "goes to completion" and has a large K. The oxidation of carbon monoxide goes to completion at 1000 K:
3. Intermediate K. Significant amounts of both reactant and product are present at equilibrium. When bromine monochloride breaks down to its elements at 1000 K, the equilibrium constant has an intermediate value: If the components of the reaction are in the same phase, the system reaches homogeneous equilibrium. If they are in different phases, the system reaches heterogeneous equilibrium. In the latter case, one or more of the components is a liquid or solid. Consider the decomposition of limestone to lime and carbon dioxide as an example of a system reaching heterogeneous equilibrium: Based on the rules we have been using for writing the reaction quotient, the expression for this reaction is: A pure solid, however, such as CaCO3 or CaO, always has the same concentration at a given temperature, that is, the same number of moles per liter of the solid, just as it has the same density at a given temperature. Moreover, since a solid's volume changes very little with temperature, its concentration also changes very little. For all intents and purposes, therefore, the concentration of a pure solid is constant. The same argument applies to the concentration of a pure liquid. Because we are concerned only with concentrations that change as they approach equilibrium, we eliminate the terms for pure liquids and solids from the reaction quotient. Thus, the equilibrium constant becomes: No matter how much CaO and CaCO3 are in the reaction vessel, so long as some of each is present, the reaction quotient for the reaction equals the CO2 pressure.
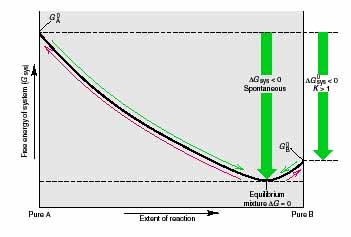
What is meant by the terms spontaneous and non-spontaneous and what is the relation to Gibbs energy? Consider the general reaction A --> B, for which K = [B]/[A] = 1; therefore, the reaction goes largely from left to right. From pure A to the equilibrium point, the reaction is spontaneous (DG < 0). From there on, the reaction is non-spontaneous (DG > 0). If we had started with pure B, the reaction would also be spontaneous until it reached equilibrium, but not thereafter. In either case, the free energy decreases as the reaction proceeds, until it reaches a minimum at the equilibrium mixture. If we start with pure A, the vessel contains mostly B when the reaction stops, so we say the forward reaction A --> B is "spontaneous." Likewise, if we start with pure B, the reaction also stops with mostly B present. Even though a small amount of A forms to satisfy the equilibrium condition, we say the reverse reaction B --> A is "non-spontaneous" because it proceeds very little in that direction. For the overall reaction A --> B (starting with all components in their standard states), G0 B is smaller than G0A , so DG0 is negative, which corresponds to K > 1 and, therefore, to a spontaneous reaction as written. Thus, the term spontaneous reaction refers to one that goes predominantly, not necessarily completely, to product.
Two ways of predicting reaction direction are from the value of DG and from the relation of Q to K. These variables are different aspects of the same principle and are related to each other by DG = RT ln Q/K. When Q = K, the system can release no more free energy. Beginning with Q at the standard state, the free energy change is DG0, and it is related to the equilibrium constant by DG0 = -RT ln K. For nonstandard conditions, DG has two components, DG0 and RT ln Q. Any non-equilibrium mixture of reactants and products moves spontaneously (DG < 0) toward the equilibrium mixture, but only when K > 1 is the reaction spontaneous for the components in their standard states (DG0 < 0). |