 *
*
|
Metal-Chelate Complexes
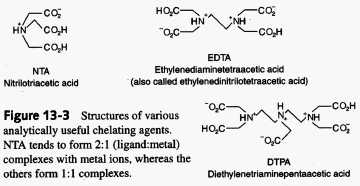
Metal ions accept electrons from electron-donating ligands. A ligand that can attach to a metal ion through one atom is referred to as being a monodentate ligand. Most transition metal ions can bind to six ligand atoms. If a ligand can bind to metal ion through more than one ligand atom it is referred to as a multidentate or chelating ligand. Multidentate ligands are able to form more stable complexes than those formed by similar mondentate ligands. This is known as the chelate effect. The chelate effect can be understood thermodynamically. Chemical reactions are driven mainly by two tendencies, an increase in entropy and/or decrease in enthalpy. Entropically, the interaction between a metal ion and a multidentate ligand would be more favorable simply because less molecules need to come together to form the complex. For example, the interaction between Cd2+ and a monodentate involves the coming together of five molecules in solution, four ligands and the Cd2+ ion, whereas the interaction of the same ion and a bidentate ligand involves three molecules, two ligands and the Cd2+ ion. If you're interested in the calculations behind complexation reactions check out this link. The overall importance of chelates is their ability to increase the solubility of ions.
 *
*One of the most commonly used chelators in analytical chemistry is EDTA or ethylenediaminetetraacetic acid. EDTA has been used to measure every element on the periodic table either by direct titration or through a sequence of indirect reactions. When EDTA complexes with a metal ion it completely surrounds the ion. The structure for this compound can be found in the above figure. Chelating agents have many possible uses in both medicine and in environmental sciences. In both areas chelates have proven to be very beneficial due to their ability to remove heavy metals from the respective systems by increasing their solubility. For more information on the therapeutic applications of chelates check out the Wellness Clinic's website.
If your're interested in some simple programs designed to calculate the speciation of a single dissolved metal in a complexing buffer out check these Buffer Calculators .
Related J. Chem. Educ. Articles:
1. Thomas J. Manning Approximating the Electrostatic Contribution
to the Entropy Change of Aqueous Phase Lanthanide-Aminocarboxylate
Complexation J. Chem. Educ. 1996 73 661. (July 1996)
2. Schwartz, Lowell M. Ion-Pair Complexation in Moderately StrongAqueous Acids J. Chem. Educ. 1995 72 823. (September 1995)
3. Po, Henry N.; Huang, Kenneth S.-C. An Inorganic Spectrophotometry Experiment for General Chemistry: A Simplified Graphical Approach for Determining Ligand-to-Metal Complexation Ratio. J. Chem. Educ. 1995 72 62.
4. Macomber, Roger S. An introduction to NMR titration for studying rapid reversible complexation. J. Chem. Educ. 1992 69 375.
5. Diederich, Francois. Molecular recognition in aqueous
solution: Supramolecular complexation and catalysis (SYMP).
J. Chem.Educ. 1990 67 813.
6. Chung, Chung-Sun. Statistical factors in complexation
reactions.J. Chem. Educ. 1985 62 107
References:
1. Drug-cyclodextrin complexation in the presence of water-soluble polymers: enhanced solubility and percutaneous transport Author: Masson, Mar, and others Source: ACS Symp. Ser. American Chemical Society 737, no. Polysaccharide Applications (1999): 24-45
2. Influence of pressure on chemical equilibria in aqueous systems - particular reference to seawater Author: Byrne, R. H., and others Source: Pure Appl. Chem. Blackwell Science Ltd. 71, no. 5 (1999): 871-890
3. Stereocontrolled synthesis and reactivity of sugar acetylenes Author: Isobe, Minoru, and others Source: Chem. Commun. (Cambridge) Royal Society of Chemistry no. 24 (1998): 2665-2676
4. Effect of acylation on flax protein functionality Author: Shahidi, F., and others Source: ACS Symp. Ser. American Chemical Society 708, no. Functional Properties of Proteins and Lipids (1998): 96-120
5. Synthetic strategies for the preparation of precursor polymers and ofmicrocapsules suitable for cellular entrapment by polyelectrolyte complexation of those polymers Author: Dyakonov, Tania A., and others Source: Ann. N. Y. Acad. Sci. New York Academy of Sciences 831, no. Bioartificial Organs (1997): 72-85
6. Covalent and noncovalent adducts of proteins with water-solublepoly(alkylene oxides) Author: Topchieva, Irina N. Source: ACS Symp. Ser. American Chemical Society 680, no. Poly(ethylene glycol) (1997): 193-206
7. Fluorescence approaches to study of protein-nucleic acid complexation Author: Hill, John J., and others Source: Methods Enzymol. Academic 278, no. FluorescenceSpectroscopy (1997): 390-416
8. Steroids in Molecular Recognition Author: Wallimann, Peter, and others Source: Chem. Rev. (Washington, D. C.) American Chemical Society 97, no. 5 (1997): 1567-1608
9. Specific problems in the measurement and interpretation of complexation phenomena in seawater Author: Byrne, Robert H. Source: Pure Appl. Chem. 68, no. 8 (1996): 1639-1656
10. Ligand-exchange chromatography of carbohydrates and glycoconjugates Author: Stefansson, Morgan, and others Source: J. Chromatogr., A 720, no. 1 + 2 (1996): 127-36
11. Thermodynamics and mechanism of complexation of peptides with 18-crown-6 in water Author: Kulikov, Oleg V., and others Source: Pure Appl. Chem. 67, no. 7 (1995): 1103-8
12. Protein-protein interaction converts a proton pump into a sensory receptor Author: Spudlich, John L. Source: Cell (Cambridge, Mass.) 79, no. 5 (1994): 747-50
13. Efficiency of best management
practices for controlling priority pollutants in runoff Author:
Scholze, Richard, and others Source: Water Sci. Technol. 28, no.
3-5, Diffuse Pollution (1993): 215-24
14. Novel formulation strategies for improving oral bioavailability
of drugs with poor membrane permeation or presystemic metabolism
Author: Aungst, Bruce J. Source: J. Pharm. Sci. 82, no. 10 (1993):
979-87
|
|
*Harris, Daniel C. Quantitative Chemical Analysis. New York: Freeman and Company 1999 (p308)